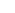Metal Electroplating 101 - Electroplating Facts & Information
 |
 |
Electroplating is one of the most common methods of metal finishing. Primarily, it is used to deposit a coating that has a plethora of different properties than those of the base metal. Contrary to popular belief, it does not involve a vat of molten metal that parts are submerged in. Rather, it is a complex process that involves a magnitude of different chemistries as well as an electrical current to deposit metal onto the surface of the part being plated. The foundation for all modern day electrochemical processes can be traced back to 1833 with the work of English scientist, Michael Faraday and his Laws of Electrolysis.
To accomplish this, the process uses a chemical solution containing the ions of the metal to be plated. Metal ions are atoms of the metal that have lost electrons and now have a positive electrical charge. Electrical current is needed and applied to the solution by a rectifier, which converts alternating current (AC) to direct current (DC). Parts are submerged into the plating solution which creates the negative electrode, commonly known as the cathode. To complete the electrical circuit, a positive electrode is needed and is called the anode. The anode is usually, but not always, made out of the pure metal being plated.
The rectifier passes direct current through the plating solution and delivers excess electrons to the surface of the cathode making it negatively charged. At the anode, metal is dissolved due to the equal loss of electrons and converted to its metal ion. These metal ions have a positive electrical charge and are naturally drawn to the excess electrons at the cathode. On the surface of the part, the metal ions are neutralized and reduced. This means they gain the negative electrons needed to once again form the stable metal atom. The metal atoms are what make up the plated deposit and will continually build in thickness depending on the amount of current and the duration for which it is applied.
Before parts can be plated, they must first go through a pre-treatment process to ensure a successful bond is made. Each base metal can require a different pre-treatment process to successfully plate it so a variety of processes must be available. Different types and degrees of oils, soils, oxides, scales, and surface imperfections existing on the parts need to be addressed before plating can occur; otherwise, parts will most likely form a weak or non-existent bond where the plating easily lifts and/or separates from the base metal. Due to this, it is extremely important to notify the plater of what soils and oxides are present on the parts that require plating. This should be done during the initial quoting process and/or whenever there is a change in the manufacturing process of the parts.
A generic pre-treat cycle typically involves these steps:
- Cleaning – This removes any oils, grease, and dirt that are on the surface of the parts. This is done through a combination of soaking and the use of a electrolytic and/or ultrasonic cleaning solution. These solutions are usually alkaline, but can be acidic.
- Rinsing – The majority of tanks in most plating shops are rinse tanks for good reason. Good rinsing is needed to completely remove the prior process’ solutions from the surface of the parts before they are further treated. Not thoroughly rinsing parts will cause contamination of the chemicals used and ruin other processes down the line. It could also create unfavorable conditions on the part surface leading to rejections.
- Pickling/Acid Dipping – Most metals have an oxide layer on their surface which would interfere with the bond and must be removed prior to plating. Acids are typically used to achieve this. Determining which acid to use and its strength will depend on the type and severity of the oxides present. Sometimes more than one acid dip is required such as when parts have heavy heat scale or rust. Mild acid dips are also used to neutralize the alkaline film that will coat parts after the cleaning process and to lightly etch the surface of the part.
- Rinsing – The same reasons apply for rinsing after acid dipping as they do for cleaning. Good rinsing is required and follows every step in the plating process to avoid contamination.
- Underplate(s) and Strikes – Many plating specifications call for the use of more than one metal to be deposited onto a part. Known as Underplates, these bottom layers of metal deposits can serve a variety of purposes depending on the finished specifications of the part. A Strike is used in certain processes to deposit a thin layer of metal that promotes adhesion of subsequent deposits.
- Rinsing – You can never have enough good clean rinsing.
- Final Plate – This is where the final visible metal is deposited onto the surface of the part.
- Final Rinsing - This is used to help ensure no plating salts or chemistry remain on the surface of the parts.
- Drying – Parts are dried in a heated atmosphere to remove any remaining moisture that may be trapped on them.
Electroless plating is similar to electroplating in that parts are submerged into a solution containing metal ions that eventually become bonded to the surface of the part. However, no electrical current is used in the process to reduce the metal ions at the cathode. Instead, a chemical called a reducing agent is incorporated into the bath chemistry and is used to supply the electrons needed for plating. Metal ions and other agents have to be routinely replenished by means of chemical additions. Unlike electroplating baths that can be purified and filtered, an electroless bath needs to be discarded and completely remade as byproducts and impurities build up after each use. Due to this, electroless plating is usually more expensive and requires greater process control. However, comparably, the electroless bath is very uniform in thickness as well as capable of producing deposits with unique and valuable properties.
There are many reasons why parts require various metals to be plated on them, but the main reason is to give the base metal different properties or dimensions that it otherwise would not have. It is actually a method of conservation that allows cheaper or more abundant materials to be used in part design while enhancing their properties with a thin coating of another metal(s).
Some of the most common properties obtained through plating are:
- corrosion resistance
- electrical conductivity
- electrical resistance
- solderability
- improved appearance
- increased value
- reflectivity
- lubricity
- anti-galling
- thermal resistance
- To act as a diffusion barrier.
Depending on the application certain metal coatings or a combination of them can achieve one or more of the desired properties listed.
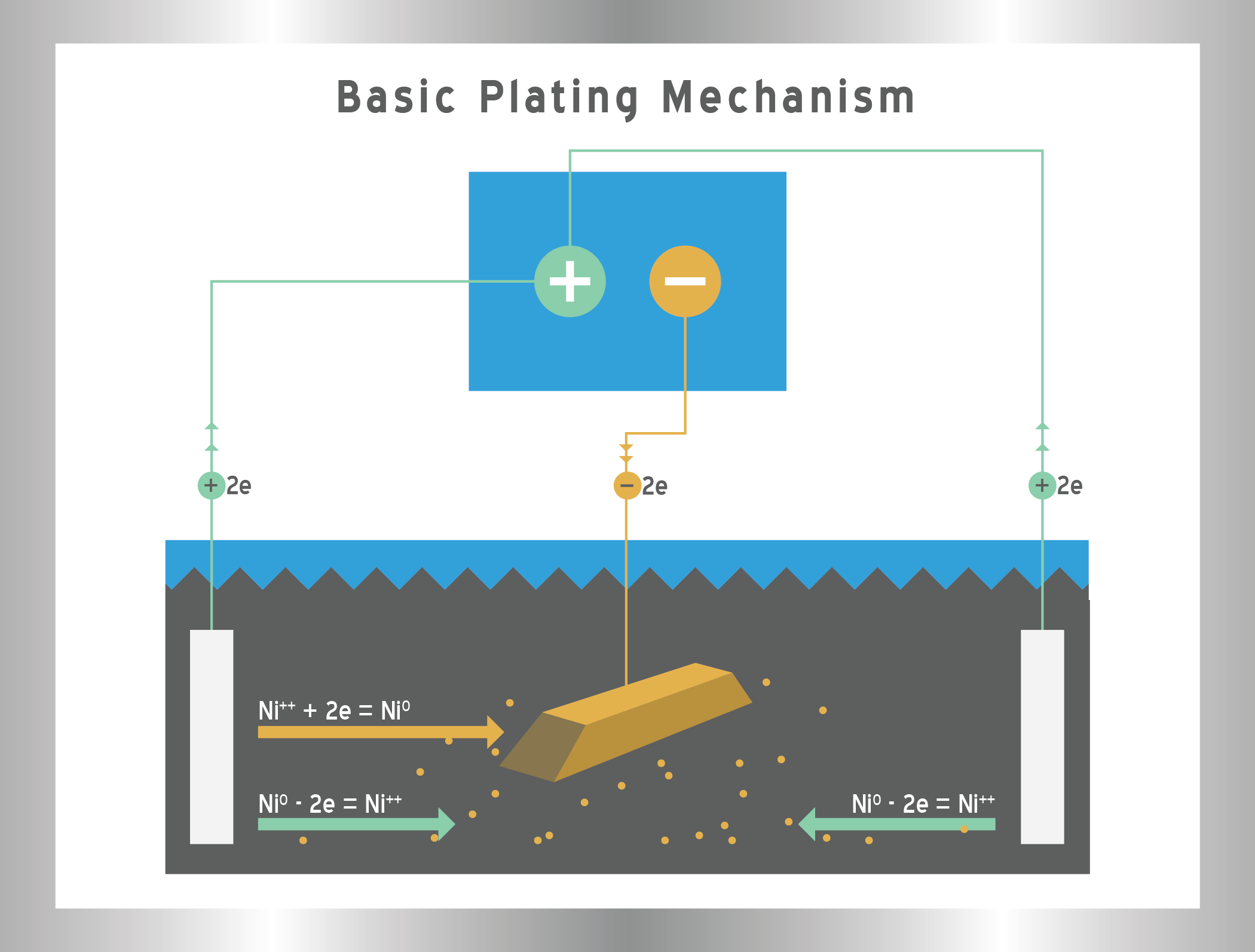
The short answer is no, electroplating does not deposit uniformly over the surface of a part and the differences can be drastic in some cases. This is due to the electrical current that is used in the process. Typically a part is centered in the middle of the plating cell surrounded by anodes on both sides. The current applied to the bath drives the metal ions to the part in flow lines called current distribution or current densities. However, electricity always takes the path of least resistance so different surface areas on the parts will have different current densities. Edges, peaks, and generally outside areas will have greater concentrations of current lines that are typically referred to as high current density areas. Those areas will receive more plating thickness. Valleys, crevices, recesses, corners, inside areas, and blind holes will have significantly less current and are called low current density areas. The difference in plating thickness can be remarkable and can vary as much as 10 times depending on the shape of the part.
Various methods can be used to increase the uniformity of the deposit. However, some can lead to additional cost and require longer processing times. If plating distribution is important, then it is imperative to let the plater know the expectations of the deposit thickness during the quoting process.
Electroless deposits are inherently more uniform because they do not rely on the use of electrical current to drive the ions to the part. Parts will plate fairly uniform as long as its surface remains wet with fresh solution and all the bath parameters are kept under control.
Many specifications and drawings will call out the thickness of plating that is to be applied. These specifications usually call for the significant surface areas of the part to meet the thicknesses. They define a significant surface as one that can be touched by a sphere with a diameter of a 0.75”. Sometimes these requirements are not practical due to the non-uniformity of the deposit caused by current densities. Therefore thickness reading locations are established and are the means by which the thickness requirements are tested. Typically critical part areas such as contact points are used for the reading locations. However, it is the platers sole discretion on where thickness readings are taken unless a reading location is designated on the part print or a certain location is discussed and agreed upon during the quoting process.
Typical thickness requirements are seen as:
- Average thickness – this means the average of all the thickness readings taken at the area the plater choses as the reading location should fall within this number +/- a given value (usually 75 micro-inches).
- Thickness range – this is a range where the average thickness of all the readings at the assigned reading location must fall into. A reading may be out of that range but is still acceptable as long as the average of all the readings fall within the range.
- Minimum thickness – this requires that no reading taken at the designated reading location shall be lower than the minimum required thickness. Any reading lower is cause for rejection. There is no maximum thickness.
- Maximum thickness - this requires that no reading taken at the designated reading location shall be higher than the maximum required thickness. Any reading higher is cause for rejection. There is no minimum thickness.
- Minimum and Maximum Range – this means that every thickness reading in a specified reading location will not be lower than the minimum stated thickness or higher than the maximum. This can be unfeasible if the range is too tight and does not allow for current density variation. Parts can have a dramatic difference in thickness depending on geometry and their position in the plating process. This range should not be interpreted as the thickness over the entire part as current densities can sometimes make this unrealistic.
Again, it is at the platers sole discretion on where thickness readings will be taken unless a required reading location(s) are established during the quoting process. We encourage you to discuss the requirements of your parts with us so everyone has the same expectations of the final plated deposit. Different reading locations may have a dramatic influence on the amount of metal that needs deposited overall. This can have a major impact on cost especially when dealing with precious metals.
The final appearance of a part will depend on a number of factors. Plating thickness is usually quite thin so the impact it has on appearance can be minimal other than color change. Typically the plated deposit will mimic the surface of the base metal. A dull rough part will probably still look dull after plating. Different bath chemistries can affect the appearance of the deposit through grain refiners and brightening agents. These create a smoother and more leveled deposit that will appear brighter and give it shine. Sometimes the use of brighteners is not desired due to various unwanted effects they could cause so a matte deposit is specified. These deposits will be dull and flat in appearance. Please keep in mind that this is not always a given. Parts that are polished or have bright underplates can still look semi-bright or bright after being plated with a matte deposit. Likewise, parts that have a rough surface may look dull after
The cost of
The approach we take is to look at the part print and discuss with our customers all the critical applications needed to successfully plate it. Every part’s surface area must be calculated prior to quoting because it is a critical factor in determining how much tank time and metal(s) will be consumed in the process.
The method of plating, usually based on part configuration or requirements, will also impact the cost. Barrel plating can usually produce more parts an hour compared to rack plating, which is more labor intensive and requires employees to rack, un-rack and potentially wire each piece. Selective plating can be one of the most costly methods since special masking and tools must be used and the masking process can sometimes be labor intensive. However, the savings in metal obtained by only
We assume all parts received or quoted are relatively clean, free of excessive scale, and in a ready-to-plate condition. Extra processing to remove heavy oils, scales, burrs and other surface conditions will require a surcharge separate from the quoted price.
The quoted price reflects only the information given to us at the time of quoting. We strongly encourage our customers to communicate all of the requirements needed to finish their parts. When a
Please keep in mind that quoting new parts is sometimes an educated guess. The plating process and how the parts react to it are a variable that remains unknown until a first article
Precious metals such as gold and silver can be volatile in price and change daily based on market conditions. Parts would have to either constantly be re-quoted or quoted with an excessively high metal price to cover any potential price swings that it would make the price unreliable and uncompetitive. To overcome the volatility of precious metals pricing, KPW uses a factor when it quotes parts. This factor is a number that can best be described as the weight in troy ounces of the metal plated and consumed during the plating process. The factor number is multiplied by the number of parts processed and the current cost of the metal. KPW notifies its customers every time there is a change in the metal’s price.
Example:
Part XYZ is quoted for gold plating with a factor of 0.300 per thousand. This means 1000 parts will consume 0.300 troy ounces of gold when they are plated. If the current price of gold is $1,200.00 per one troy ounce then the price to plate 1000 pcs is:
$1,200.00 x 0.300 = $360.00 of gold that will be billed separately per every 1000 pcs plated.
So, if a job consisting of 6,750 pcs of XYZ are plated then:
6.750 (because this factor is per 1000 pcs) x $360.00 = $2,430.00 gold consumed and billed separately for the entire job.
This depends entirely on what is required of the finished parts and the physical characteristics of them. Different coatings and combinations of coatings provide different properties and typically a compromise is given between the best benefits various coatings offer and cost. Engineers will typically specify the plating requirements on a drawing or reference an appropriate military or government spec. We encourage our customers to ask questions and discuss the requirements of the deposit(s). We can help in the selection of coatings to use and what to expect from them to ensure the part is a success.
The method that's used to process parts will depend on the physical characteristics and any special requirements they have. Small parts that tumble freely are ideal candidates for barrel plating while larger (typically longer than 2”) and/or heavier parts will probably have to be rack plated. Soft materials may be dinged or slightly damaged in the barrel process due to the tumbling action, so rack plating might be the only option to successfully plate them. We will look at the characteristics of the parts during the quoting process and determine which method is most appropriate to process them with. Customers always have final say and can specify which method to use. We are happy to discuss the various plating options available and what impact they may have.
The plating process is dynamic and contains many constantly changing variables that could potentially lead to a defect in the deposit. Common defects include:
- Lack of Adhesion
- Roughness
- Dullness or Hazes
- Stains
- Etching or Pitting
- Out of Tolerance Dimensions
- Cracking
- Spotting out or Bleed Out
Bath chemistries, cleaners, acids, and rinses all need daily maintenance. Chemical additions have to be made to them, contaminates must be removed, and various parameters such as temperature must constantly be controlled. Equipment must also be calibrated and maintained on a continual schedule to ensure its proper functioning. Any one area can give rise to a defect. For example, the entire process might be perfect except for one rinse having a little oil on its surface and that is enough to ruin the outcome. Operator error is also an area that needs to be taken into consideration. An operator who uses the wrong process will have little chance of successfully plating a part.
KPW maintains an in-house laboratory to monitor, test, and maintain its processes on a daily basis. Employees are trained to look for inconsistencies and notify management and soon as one is discovered. Employees receive training in the plating process and how to properly maintain equipment. Standard plating procedures are established for every part KPW processes and records are maintained for accuracy and reference. Every job is inspected to ensure they meet their established standards. Every plater will occasionally have defective parts due to the inherent complexity of the process. KPW spends an extraordinary amount of time and resources on its employees, processes, and equipment to help minimize any potential problems that might interfere with part quality.
It is obvious that many variables in the plating process can give rise to a plating defect, but what is sometimes not so obvious is that the part(s) itself could be the reason for why the plating failed. Surface defects resulting from the manufacturing process can be the actual root cause to a plating problem. Some examples of part defects that potentially lead to plating defects are:
- Parts with rolled over surfaces, metal tears, scratches, shavings, and burrs could all trap dirt, oils, and chemical solutions. These microscopic defects can lead to disastrous results if not known and fixed before plating.
- Oil from parts that are heat-treated can be baked onto the surface of the parts making them extremely difficult to remove.
- Excessive heat scale or rust can lead to a lack of adhesion in the plated deposit. Usually strong acids need to be used to remove them, which could impact the part surface and dimensions as well as open up other defects such as porosity in the underlying metal.
- Porosity, pits, or cracks in the base metal. These can entrap solutions that later bleed out after the plating process is complete. Capillary action can actually “suck” solutions into these pores making it extremely difficult, if not impossible, to properly rinse afterwards.
- Residual flux from soldering operations can be difficult to remove and could result in adhesion failures.
- Not using or informing the plater of the correct base metal. It is very important for us to know exactly what the material is since many different substrates require specific pre-treatments to properly plate them. Not informing the plater of the actual base metal can lead to a host of defects. An example would be stating the material is just copper rather than tellurium copper, which requires a very specific process to successfully plate.
KPW’s processes are designed to overcome many of these potential problems and produce a high quality coating. Communicating any potential material problems with us is important and can help ensure the success of the parts.
This is absolutely untrue if a plating company is doing the right things. It is true that plating processes are required to use many hazardous materials such as acids and cyanides because they are necessities in the electrochemical process. These solutions can become hazardous waste once they are spent and the potential for pollution is present for the unscrupulous. However, plating companies are held to strict Federal, State, and Local requirements such as the Clean Water Act as well as discharge limits that are monitored and enforced.
Klein Plating has invested hundreds of thousands of dollars into its waste treatment facilities to ensure compliance that goes above and beyond these regulations. Hazardous materials are removed from our process waters before they are discharged and all solid hazardous materials generated by our operations are sent out to EPA licensed facilities for reclaim and incineration. Nothing is landfilled. We invest a lot in and take great pride and effort in our waste treatment facilities. After all, we are part of this community and drink, enjoy, and bathe in the same water as everyone else.
 Klein Plating Works has done an outstanding job for us in the past and I know that I can count on them for future orders.
Klein Plating Works has done an outstanding job for us in the past and I know that I can count on them for future orders.